The presence of the SARS-Cov-2 virus in saliva has been demonstrated in various scientific papers as well as in symptomatic patients, even in asymptomatic or pre-symptomatic patients. The saliva sample can be considered an option for the detection of SARSCoV-2 infection if it is not possible to obtain nasopharyngeal swabs.
As for swabs, also for salivary tests there are molecular type tests (which detect the presence of the virus RNA in the sample) and third generation laboratory antigenic type tests (which detect viral proteins in the sample). The collection of saliva is undoubtedly simpler and less invasive than the nasopharyngeal swab, therefore, thanks to these characteristics this type of test could be manageable and easily usable also for the screening of large numbers of samples, in contexts for which the times to obtain results are compatible with processing in the laboratory, as, for example, for low-risk school communities.
MOLECULAR SALIVARY TEST RT-PCR WITH QR CODE
Molecular RT-PCR examination with QR CODE for the search for the genetic material (RNA) of SARS-CoV-2.
RESEARCH OF 4 GENES: Geni Rdrp / S, Gene N and Gene E.
cost € 60 – response within 24 hours from Monday to Friday, weekends and holidays 48 hours
THIRD GENERATION SALIVARY ANTIGENIC TEST
The detection of SARS-CoV-2 antigens (third generation laboratory salivary antigenic) is performed in the laboratory with a quantitative dosing system, expressed in pg / ml, fully automated on the basis of CLEIA technology (ChemiLuminescent Enzyme Immunoassay). The detection of the SARS-CoV-2 virus nucleocapsid protein (NP) detected by the system as per the stated clinical performance data demonstrates excellent correlation with the RT-PCR method and it is also shown that the detection capability of the assay is not theoretically influenced by the new variants of the virus; the manufacturer declares a sensitivity (agreement with positive) of 100% and specificity (agreement with negative) of 99.3%.
cost € 22 – reply within 12 h
SALIVARY TEST FOR SYMPTOMS
Saliva can be used for symptomatic patients as an alternative to gold / nasopharyngeal swabs for the identification of SARS-CoV-2 infection, preferably within the first 5 days from the onset of symptoms.
SALIVARY TEST FOR ASYMPTOMATICS
The saliva sample can be considered an option for detecting SARS-CoV-2 infection in asymptomatic individuals undergoing repeated screening for professional or other reasons, to increase the acceptability of repeated tests, in particular:
-if they are subjected to screening very elderly or disabled individuals
-in case of shortage of
-tampons testing in children.
METHOD OF COLLECTING THE SAMPLE
The use of saliva for the diagnosis of SARS-CoV-2 infection involves a non-invasive collection method, however to obtain an adequate sample some precautions are necessary: saliva must be collected fasting, without smoking, brushing the teeth , drank, used chewing gum and preferably in the morning.
If it is not possible to collect the saliva sample in the morning, it can be collected during the day not before 30 minutes have passed since food or drink.
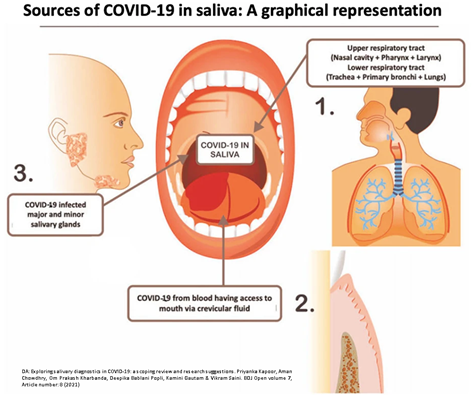
The figure * shows the ways in which the virus reaches the saliva: from the upper respiratory tract, from the lower one, from the salivary glands and from the blood through the vessels in the crevicular fluid.
With reference to the provisions of Circular no. 0221675 of 14/05/2021 of the Ministry of Health on the use of saliva for the diagnosis of SARS-CoV-2 infection, we inform you that:
- the molecular test on nasopharyngeal and oropharyngeal sample represents the international gold standard for the diagnosis of COVID-19 in terms of sensitivity and specificity. The real-time RT-PCR (Reverse Transcription-Polymerase Chain Reaction) method, which is the most widespread among molecular tests, allows, through the amplification of the most expressed viral genes, to detect the presence of the viral genome as well as in symptomatic subjects, even in the presence of low viral load, often pre-symptomatic or asymptomatic;
- the saliva sample can be considered an option for the detection of SARS-CoV-2 infection if it is not possible to obtain gold / nasopharyngeal swabs:
- in symptomatic cases, the test must be performed within the first 5 days from the onset of symptoms:
- in asymptomatic patients, the test can be considered an option, to nasopharyngeal swab, for those who are repeatedly screened for professional reasons or to increase the acceptability of repeated tests: very elderly and disabled individuals, lack of swabs; tests for children: Saliva tests can be a useful tool for monitoring SARS-CoV-2 infection in children.
According to the provisions of the regional determination of 29 September 2020 No. G11083, the positivity to this test must in any case be confirmed by the search for viral RNA by RT-PCR. The prescription of the test substantiates the diagnostic suspicion and therefore it will be mandatory to report to the public health and hygiene service (SISP) competent for residence and domicile and at the same time they will be included in the regional platform of the COVID body. If positive, the general practitioner should be contacted.